Description
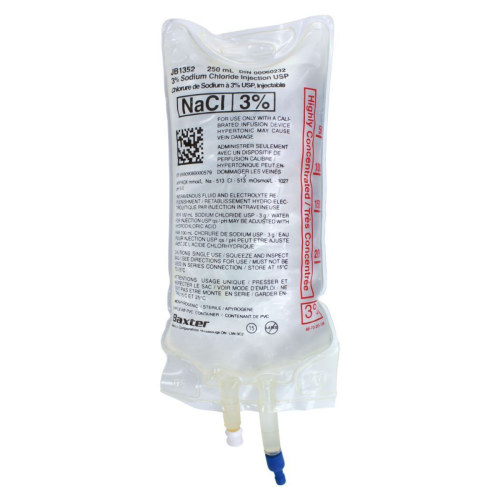
The Baxter 3% Sodium Chloride Injection Solution USP is a sterile, intravenous hypertonic saline solution designed for medical use in the management of electrolyte imbalances and fluid replacement therapy. Supplied in convenient 250 mL bags and available in a case of 30, this solution provides clinicians with a reliable and ready-to-use option for hospital and clinical settings.
Formulated to USP standards, Baxter’s 3% sodium chloride injection is ideal for correcting hyponatremia, maintaining plasma sodium levels, or providing hypertonic therapy under the supervision of qualified healthcare professionals. Its IV-ready formulation ensures consistent, sterile delivery for patient safety and therapeutic efficacy.
Key Features at a Glance
- Concentration: 3% Sodium Chloride (hypertonic saline)
- Volume: 250 mL per bag
- Sterility: Sterile for intravenous use
- Packaging: Case of 30 for high-volume clinical use
- USP-grade formulation ensures purity and reliability
- IV-ready solution for fluid and electrolyte management
Ideal Uses
- Treatment of hyponatremia and low plasma sodium levels
- Hypertonic therapy in critical care and hospital settings
- Electrolyte and fluid replacement
- Suitable for use in emergency medicine, surgical, and inpatient care
The Baxter 3% Sodium Chloride Injection Solution USP – 250 mL delivers precise, sterile, and ready-to-use hypertonic saline for intravenous therapy. Its convenient packaging and USP-grade quality make it an essential supply for hospitals, critical care units, and other healthcare facilities requiring safe and effective sodium replacement therapy.
Specifications
Baxter
Sodium Chloride IV Bags



